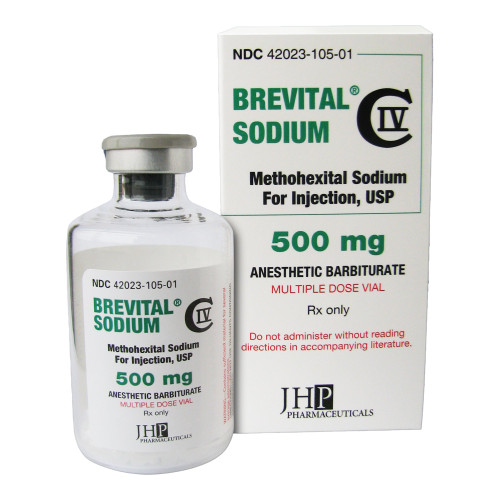
Brevital® Methohexital Sodium 500 mg / 50 mL Injection Multiple-Dose Vial 50 mL CIV
BREVITAL, VL 500MG/50ML 50ML CIV
Controlled Substance: Controlled substances ship separately.
Features
- Store at controlled room temperature 20 to 25 degrees C (68° to 77°F)
- For Intravenous Use in Adults
- For Rectal and Intramuscular Use Only in Pediatric Patients
- With 30 mg anhydrous sodium carbonate
Product Specifications
| McKesson # | 668506 |
|---|---|
| Manufacturer # | 42023010501 |
| Brand | Brevital® |
| Manufacturer | Par Sterile Products LLC |
| Country of Origin | Unknown |
| Alternate Manufacturer Number | 1888817 |
| Application | Barbiturate (General Anesthetic) |
| Container Type | Multiple-Dose Vial |
| Dosage Form | Injection |
| Drug Schedule | CIV |
| Generic Drug Code | 12801 |
| Generic Drug Name | Methohexital Sodium |
| NDC Number | 42023010501 |
| Product Dating | McKesson Acceptable Dating: we will ship >= 90 days |
| Strength | 500 mg / 50 mL |
| Type | Intravenous |
| UNSPSC Code | 51272005 |
| Volume | 50 mL |